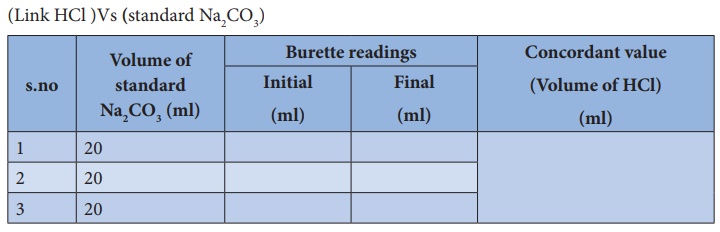
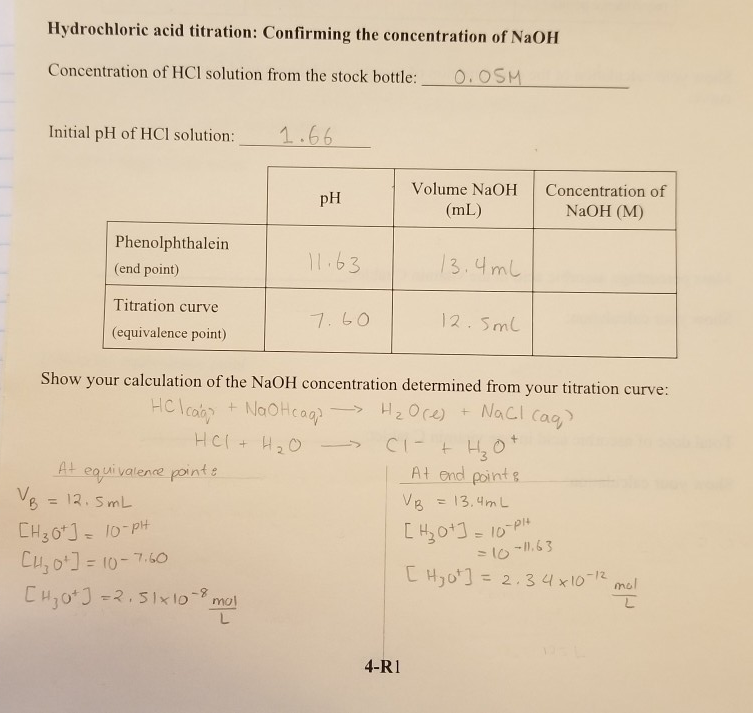
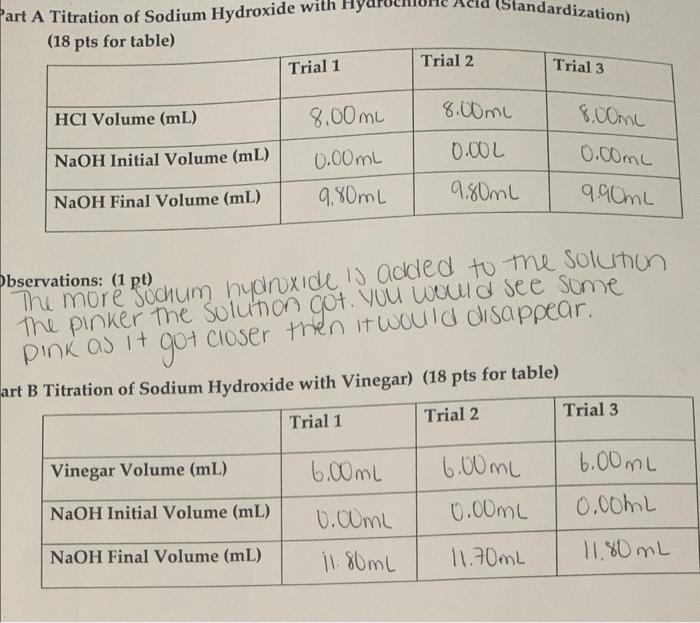
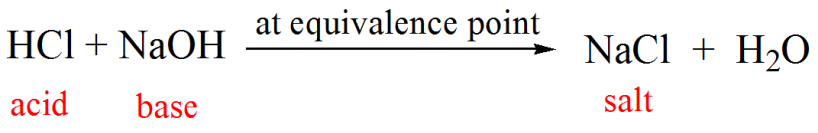A student performs a second titration in which the student titrates a 20. mL sample of 0.20 M HCl(aq) with 0.10 M NaOH(aq). How many ml of 0.10 M NaOH(aq) would be
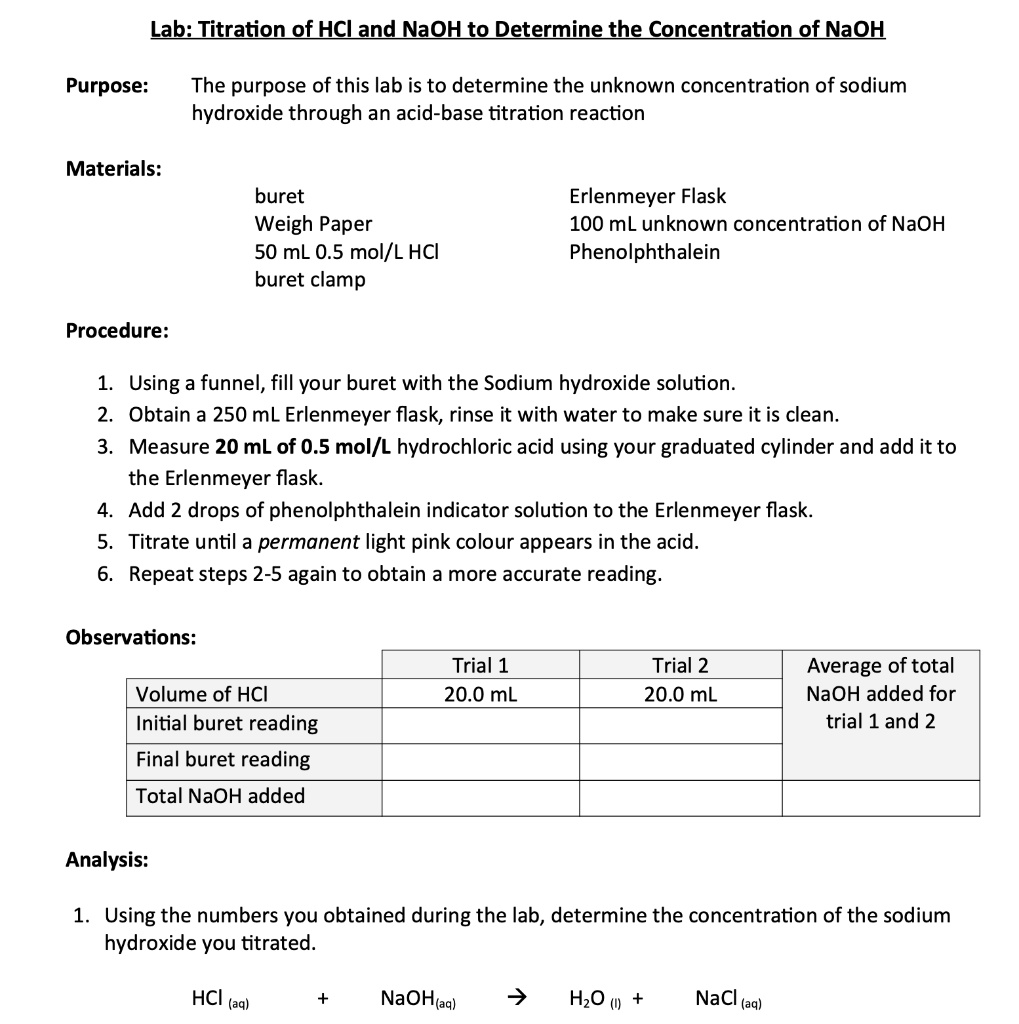
SOLVED: Lab: Titration of HCLand NaOHto Determine the Concentration of NaOH Purpose: The purpose of this lab is to determine the unknown concentration of sodium hydroxide through an acid-base titration reaction Materials:

SOLVED: 0.200 M sodium hydroxide (NaOH) being added to 30 mL of hydrochloric acid (HCl) of unknown concentration. Your goal is to measure the volume of sodium hydroxide needed to neutralize the

Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.
What volume of 0.1mol/dm3 hydrochloric acid will be required to neutralize 20cm3 of 2.0mol/DM3 sodium hydroxide? - Quora

Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

Question Video: Calculating the Concentration of a Hydrochloric Acid Solution Using Experimental Data | Nagwa